
It is a diatomic nonpolar molecule with a bond angle of 180 degrees. There is a triple bond between both nitrogen atoms.Įach N is surrounded by two dots, which are called lone pairs of electrons. N2 dot structure would comprise two atoms of nitrogen (N) atoms. Explain the N2 dot structure in the simplest form That’s why nitrous oxide is called laughing gas. Hence, nitrogen oxide is also known as “laughing gas”. If a mixture of nitrogen oxide and a little oxygen is inhaled for a sufficiently long time, it produces hysterical laughter. Why Is Nitrous Oxide Called Laughing Gas? When electrons are shared equally between atoms in a diatomic molecule or when polar bonds in a larger molecule cancel each other out, nonpolar molecules form. Polar When the electronegativity of the bonded atoms differs, molecules are formed.
#N2 molar mass free
If you have any other questions, please feel free to comment.

Some of the frequently asked questions are given below. The molar mass of nitrogen is 28 atomic mass units (AMU).
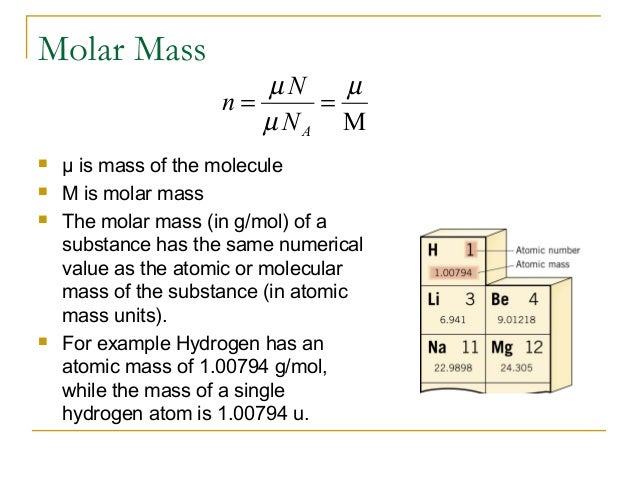
N2 is a nonpolar molecule with linear geometry.The bond angle is 180 degrees, and there are 10 valence electrons.In a nitrogen molecule, a triple covalent bond is represented by three lines between two atoms of nitrogen.To summarize everything in this article, the following are some important points: In its combined state, nitrogen is found in all living matter, including, animals and plants, in the form of proteins, urea, and amino acids.Inorganic compounds of nitrogen are not commonly found as minerals.N 2 is an inactive gas in comparison with oxygen, which is the next major constituent of air.It is a nonmetal and a poor conductor of heat and electricity.Nitrogen is present in the free state in the air as a major constituent (78% by volume).The molecular mass of a nitrogen molecule (N 2) = 14.01 u + 14.01 u = 28.02 u. Nitrogen is a chemical element with the symbol N, atomic number 7, and atomic mass of 14.00674 u.įor nitrogen, the mass of the N 2 molecule is



 0 kommentar(er)
0 kommentar(er)
